- Singapore 바카라사이트 승리바카라 소울카지노 production facility set to be operational in H1 2025 – Competitive advantage through vertical integration of 바카라사이트 승리바카라 소울카지노 development
- 96% purity in DAR 바카라사이트 승리바카라 소울카지노 achieved – ‘X-Link’ technology ensures 21-day blood stability
- Strengthening collaboration with LigaChem Biosciences and AbTis – “Partnering for co-development of technology in 바카라사이트 승리바카라 소울카지노”

[by Ji, Yong Jun] WuXi XDC is jumping into the antibody drug conjugate (ADC) contract research, development, and manufacturing (CRDMO) sector. The company recently announced plans to strengthen its technology collaboration and co-development partnership with Korean biotechnology firms, leveraging its ADC manufacturing facility in Singapore, set to be operational in the first half of this year. This strategic initiative aims to capture the growing domestic demand for ADC development by offering a comprehensive ‘vertical CRDMO service’ that can provide integrated services from antibody discovery to conjugation technology, process development, and commercial-scale production.
바카라사이트 승리바카라 소울카지노 XDC hosted the ‘Ignite Future CRDMO DAY in Seoul Conference’ for Korean biotechnology companies on May 9 at the Hotel Sofitel Ambassador Seoul in Jamsil, Songpa District, Seoul. During the event, the company highlighted its upcoming ADC manufacturing facility in Singapore, slated to begin operations in the first half of this year, and emphasized its commitment to positioning Korea as a strategic technology partner, rather than just a customer.
바카라사이트 승리바카라 소울카지노 XDC, a specialized ADC CRDMO subsidiary of 바카라사이트 승리바카라 소울카지노 Biologics founded in 2021, has completed over 900 projects in collaboration with more than 500 global partners. To date, the company has submitted 85 investigational new drug (IND) applications.
Its first commercial ADC manufacturing facility in Singapore's Tuas Biomedical Park is set to be operational this year, with a production capacity of up to 8 million vials annually. At the event, Jun Hu, Vice President of 바카라사이트 승리바카라 소울카지노 XDC, stated, "The Singapore campus, located in a major cluster for global pharmaceutical companies, is positioned to serve as a key global base for ADC manufacturing."
Antibody-drug conjugate (바카라사이트 승리바카라 소울카지노) is one of the emerging 'new modalities’ (a new approach to new drug development), gaining significant attention worldwide. The 바카라사이트 승리바카라 소울카지노 market is projected to expand over fivefold, from approximately USD 13.2 billion (KRW 18.4734 trillion) in 2024 to USD 66 billion by 2030. “Around 60% of 바카라사이트 승리바카라 소울카지노 developers currently partner with contract development and manufacturing organizations (CDMOs),” Jun explained. “Ultimately, this trend is likely to drive continued growth in demand for 바카라사이트 승리바카라 소울카지노 CDMO services.”
WuXi XDC places a strong emphasis on providing comprehensive ‘vertical services.’ “ADC development traditionally involves multiple vendors for processes such as antibody production, linker and payload synthesis, conjugation, formulation, and analytical testing,” Jun emphasized. “All of these processes can be managed within a single platform.”
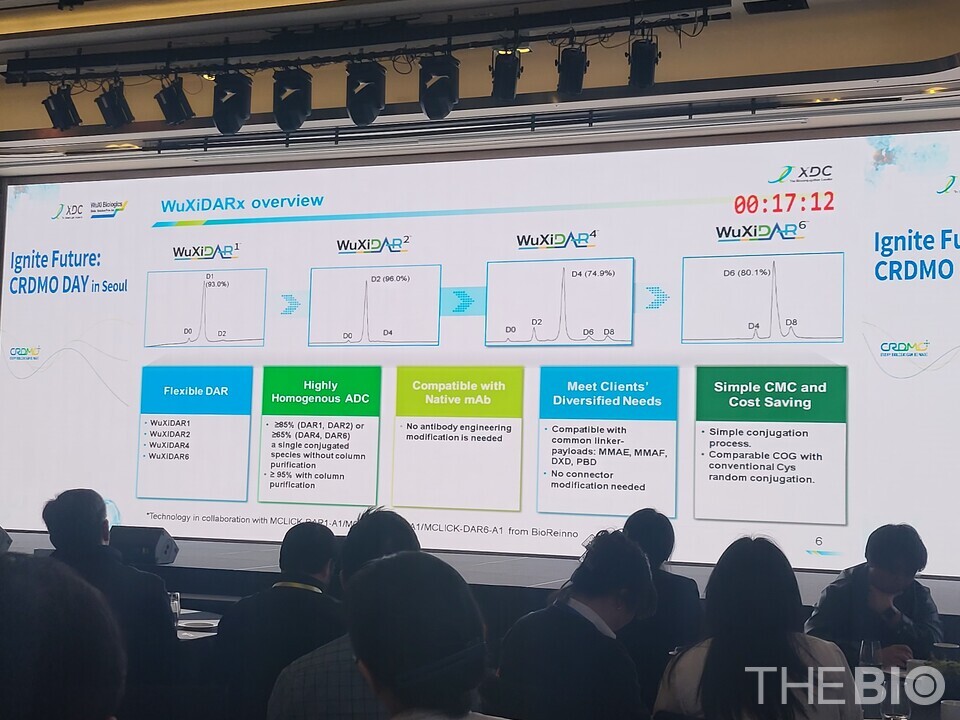
바카라사이트 승리바카라 소울카지노 XDC has developed two specialized platforms: ‘바카라사이트 승리바카라 소울카지노DARxTM’ which allows precise control of the ‘Drug-to-Antibody Ratio (DAR)’ without requiring antibody modification, and ‘X-Link,’ a linker platform. According to the company, 바카라사이트 승리바카라 소울카지노DARx can produce ADCs with target DAR values (e.g., 4, 8) with production efficiencies of 90-96% or higher. The X-Link platform offers the advantage of providing blood stability for over 21 days and completing conjugation in less than an hour, significantly surpassing the capabilities of traditional MS-PEG series linkers.
“The use of ‘바카라사이트 승리바카라 소울카지노 Direct’ enables high-precision conjugation of DAR values of 4 or higher without the need for genetic modification of antibodies,” expressed Jiawei Lu, Head of Bioconjugation Discovery Service at 바카라사이트 승리바카라 소울카지노 XDC. DAR (Drug-to-Antibody Ratio) refers to the average number of drug molecules (small-molecular-weight toxic agents) attached to each antibody in an ADC. For instance, a DAR of 4 indicates that, on average, four drug molecules are conjugated to each antibody.
바카라사이트 승리바카라 소울카지노 XDC outlined its strategic vision to strengthen technology co-development partnerships with Korea, leveraging the upcoming launch of its ADC production facility in Singapore. The company aims to position itself as a partner supporting the development and production of ADCs alongside Korean biotechnology companies.
바카라사이트 승리바카라 소울카지노 XDC has formed strategic ADC development partnerships with Korean biotech companies, including LigaChem Biosciences (hereinafter referred to as LigaChem Bio), AbTis, and Celltrion. In February, the company signed an agreement with LigaChem Bio to expand the scope of co-development for ADC and is currently incorporating AbTis’ linker purification technology as a critical component of its manufacturing process.
“In addition to technology transfer between China and Singapore, we have extensive experience in supporting the relocation of our clients’ production bases to 바카라사이트 승리바카라 소울카지노,” said Xudong Dai, head of Bioconjugation Process Development at 바카라사이트 승리바카라 소울카지노 XDC. “This capability enables us to effectively minimize the risks associated with the relocation process,” he added.

