[Interview] 바카라 사이트 추천디시 Soon-gyu, Co-CEO of Avelos Therapeutics

[by Lee, Young Sung] Avelos Therapeutics (hereinafter referred to as Avelos), a biotechnology firm specializing in the development of anticancer drugs, has disclosed preclinical data on two assets targeting the DNA damage response (DDR) field, expressing confidence in the potential for future technology licensing opportunities.
Based on 바카라 사이트 추천디시 indicating that the novel drug candidates considerably increased anticancer efficacy in combination with existing treatments compared to competing drugs, the company is committed to amplifying the value of these assets through future clinical trials.
바카라 사이트 추천디시 is prioritizing the development of small-molecule synthetic novel drugs in the areas of DDR, synthetic lethality, and cell cycle regulation.
바카라 사이트 추천디시 Soon-gyu, co-CEO of Avelos, recently conducted an interview with <THE BIO at the company’s headquarters located in Magok-dong, Gangseo District, Seoul.
"Over the past three and a half years, we have concentrated on building our reputation as a solid emerging biotech company by establishing a research and development foundation in collaboration with CEO Park Young-whan and securing a promising pipeline," 바카라 사이트 추천디시 said. "With our first drug candidate advancing into clinical trials, we will now focus on generating research and development outcomes as well as commercialization achievements, with the goal of growing into a global biotech company specializing in synthetic lethal drugs."
Of the five pipelines at 바카라 사이트 추천디시, the asset demonstrating the fastest development progress is designated as 'AD1208 (Project name: AVS1001)'.

◇"MASTL kinase inhibitor 바카라 사이트 추천디시’s phase 1b/2a single and combination clinical trials scheduled for H2 2026"
바카라 사이트 추천디시 represents the first inhibitor in the series targeting 'MASTL (microtubule-associated serine/threonine kinase-like) kinase, which plays a role in the cell cycle and DDR.
In diverse cellular environments, DNA can undergo breaks in one or both strands of its double helix structure. In such instances, the cell must effectively repair the damage to preserve genomic integrity. Failure to do so results in either cell death or abnormal cell proliferation, potentially leading to the formation of cancer cells. 바카라 사이트 추천디시 acts by inhibiting the division of these mutated cancer cells while concurrently disrupting their DNA repair mechanisms, ultimately inducing cancer cell death.
바카라 사이트 추천디시 obtained approval from the Ministry of Food and Drug Safety in December 2024 and is presently conducting a phase 1a clinical trial of AD1208 in patients with solid tumors. This trial is taking place at Samsung Medical Center, Seoul National University Bundang Hospital, and Severance Hospital, with preparations underway for Cohort 3. Since the first administration to a patient in early March of this year, no drug-specific abnormalities have been observed.
AD1208 is designed to target various solid tumors, including colon cancer, breast cancer, and sarcoma. 바카라 사이트 추천디시 provided an overview of the early development stage, noting, "In animal studies using colon cancer models, AD1208 demonstrate d substantial efficacy as a standalone cell division inhibitor," and further remarked, "When administered at a low dose in combination with an approved drug, we anticipated minimizing general 'toxicity' signals while maximizing the synergistic effects of the combination therapy."
"In fact, AD1208 demonstrated a superior anticancer effect in combination therapy with either 'FOLFOX/FOLFIRI' or 'genetically targeted therapy approved based on genetic testing' compared to single administration in conventional chemotherapy," 바카라 사이트 추천디시 said. FOLFOX consists of a combination of 'folinic acid (Leucovorin),' the antimetabolite '5-FU (5-fluorouracil),' and 'oxaliplatin.'
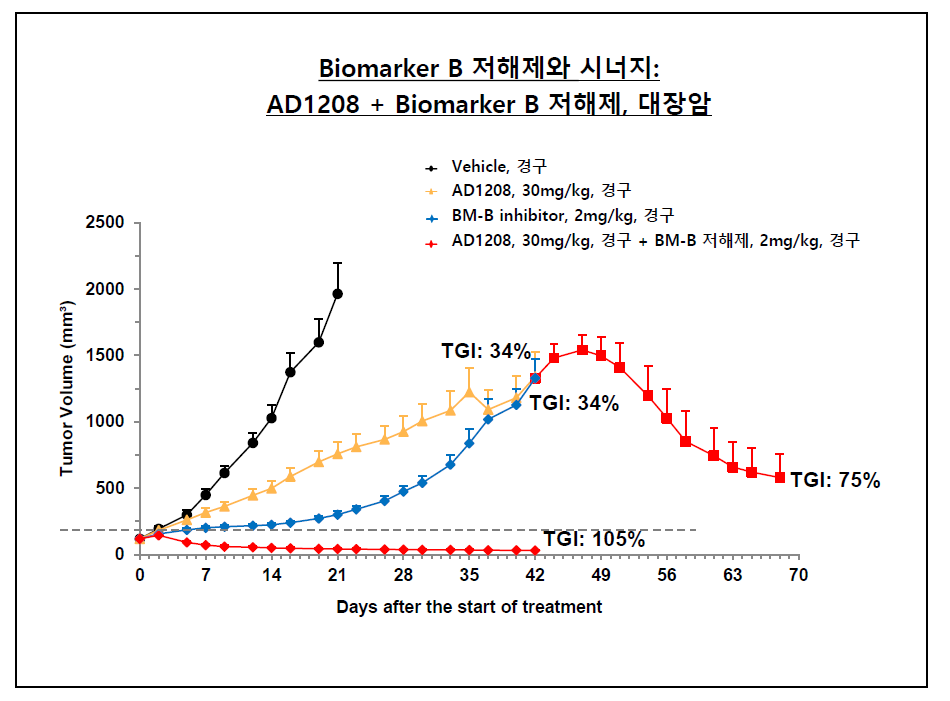
Furthermore, according to 바카라 사이트 추천디시, AD1208 exhibited superior efficacy compared to monotherapy with PARP inhibitors in other animal model studies when administered in combination with PARP inhibitors (approved). Its therapeutic effects were also validated in animal experiments on colon cancer, where AD1208 was administered in combination with treatments targeting biochemical (mitogenic) signals that induce cell division.
바카라 사이트 추천디시 described the effects of AD1208, noting, "When AD1208 was administered to an animal model that had acquired resistance to an already approved second-line therapy, there was a significant reduction in tumor size. Moreover, in an animal model treated with AD1208 in combination with the second-line treatment from the onset of the experiment, the tumor size consistently remained at a minimal level throughout the course of the study." 바카라 사이트 추천디시 further elaborated, "We anticipate the synergistic potential of AD1208 in combination therapies and are developing it as a 'biomarker-driven' treatment, establishing our research and development strategy around a specific biomarker."
Additionally, AD1208 demonstrated a clear advantage over competing drug candidates as a 'monotherapy' in an animal model of castration-resistant prostate cancer (CRPC). "When administered alone in cases of prostate cancer resistant to docetaxel, AD1208 exhibited a relatively higher cell division inhibition rate compared to the competing drug," 바카라 사이트 추천디시 highlighted. "In prostate cancer patients already resistant to chemotherapy, the target of AD1208, MASTL, is overexpressed."
According to Avelos, the phase 1a clinical trial application for AD1208 was submitted to the Ministry of Food and Drug Safety in October of last year, and despite being a first-in-class new drug, it was rapidly approved in December. "We expect favorable results in terms of safety and efficacy," 바카라 사이트 추천디시 remarked. "Phase 1a is projected to conclude in the second half of 2026," he added. "AD1208's Phase 1a clinical trial (in solid tumor patients) is currently progressing through the second of six cohorts, with no significant toxicity issues reported to date. If Phase 1a is successfully completed, we intend to determine the optimal dose and combination partner, based on the results of preclinical animal studies, and proceed with Phase 1b/2a clinical trials as both a monotherapy and in combination in the second half of next year."
◇"USP1 inhibitor 바카라 사이트 추천디시 underway… Expected to surpass Roche's competing drug"
바카라 사이트 추천디시 is advancing 'AVS1004 (lead compound: AD0126)', a USP1 (ubiquitin-specific protease 1) inhibitor project, as its second major asset.
바카라 사이트 추천디시 is a deubiquitinating enzyme that facilitates DNA repair by removing ubiquitination from key DNA repair proteins like FANCD2, PCNA, and BRCA1/2.
When the USP1 enzyme is inhibited, the ubiquitination of these DNA repair proteins remains intact, leading to their degradation and a subsequent reduction in DNA repair capacity. This mechanism ultimately results in the death of 바카라 사이트 추천디시 cells that have defects in DNA repair.
In particular, given that USP1 inhibitors demonstrate a synergistic effect when used in combination with PARP inhibitors, 바카라 사이트 추천디시 is developing a pipeline that integrates its USP1 inhibitor (AD0126), currently under development, with PARP inhibitors.
"PARP inhibitors also exert a synergistic effect on cancer cells. They simultaneously inhibit the DNA damage repair mechanism alongside AD0126, which is why an increasing number of companies are pursuing the development of USP1 inhibitors for combination therapy," 바카라 사이트 추천디시 stated. Currently, multinational pharmaceutical companies, including Roche, DeBiopharm, and Simcere, are also engaged in the development of USP1 inhibitors.
"Toxicity testing can be a significant obstacle in the development of USP1 inhibitors," 바카라 사이트 추천디시 said. "We are dedicated to identifying the optimal candidate by performing preliminary toxicity testing against competing substances ahead of time."
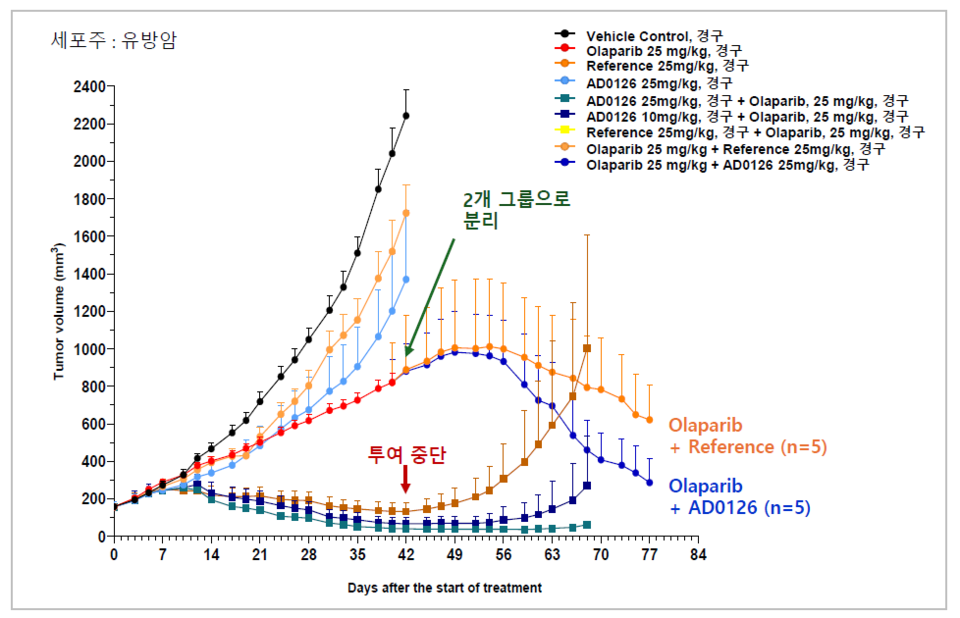
Among the lead compounds evaluated to date, AD0126 demonstrated a much higher cancer suppression rate than its competitors in animal models of breast cancer. "When AD0126 was administered in combination with a PARP inhibitor, it exhibited a cancer growth inhibition effect approximately three to five times greater than that observed with KSQ-4279, a compound developed by Roche, when similarly combined with a PARP inhibitor," 바카라 사이트 추천디시 further explained.
"The PARP inhibitor group exhibits a limited cancer growth suppression rate of about 50% by around day 42 of the animal efficacy studies, as resistance typically develops," 바카라 사이트 추천디시 further noted. "At this stage, combining USP1 inhibitors can help overcome resistance and strengthen cancer growth suppression. Notably, when AD0126 and Roche's KSQ-4279 were co-administered, the cancer growth inhibition effect of the AD0126 group was confirmed to be significantly superior in comparison," he added.
In particular, Avelos is also conducting research on novel target tasks that could maximize the synergistic effects when combining AD0126 with drugs that operate with different mechanisms of action, thereby aiming to achieve superior efficacy. “We are pursuing this research to secure marketability and competitiveness, ultimately striving for efficacy that surpasses that of the combination of PARP inhibitors and USP1 inhibitors,” 바카라 사이트 추천디시 emphasized.
◇“Exploring the incorporation of ‘바카라 사이트 추천디시’ treatment into ADC payload”
바카라 사이트 추천디시’ third project involves the early-stage development of antibody-drug conjugates (ADCs), a highly promising modality in the market. Notably, there is growing interest in efforts to use DDR- or cell cycle control-targeting treatments as ADC payloads or to use them in combination with ADCs to enhance therapeutic efficacy.
This approach is grounded in the principle that existing ADCs induce DNA damage responses, and by combining them with DDR-targeting treatment, it is possible to either increase the efficacy of 바카라 사이트 추천디시 cell killing by inhibiting DNA repair processes or to overcome ADC resistance.
"In line with this trend, Avelos is actively engaged in discussions for joint research collaborations with companies specializing in ADCs, aiming to utilize its anticancer compounds under development as novel ADC payloads," 바카라 사이트 추천디시 remarked.
바카라 사이트 추천디시 is currently seeking global technology transfer and joint research partners for its drug candidates under development. At the BIO Korea event held in Korea last May, the company held partnering meetings with several global pharmaceutical and biotechnology companies. The company is also determined to participate in BIO USA in June to explore global licensing opportunities.
"We intend to conduct a technology evaluation by the end of 2026 and subsequently pursue a listing on the KOSDAQ market after 2027," 바카라 사이트 추천디시 stated.
Conversely, 바카라 사이트 추천디시 secured a Series B investment totaling KRW 17 billion (approximately USD 12.3 million) in April 2024. Earlier this year, the company received a strategic investment from Hyundai Pharmaceutical.

